| Channel | Publish Date | Thumbnail & View Count | Download Video |
|---|---|---|---|
| | Publish Date not found | 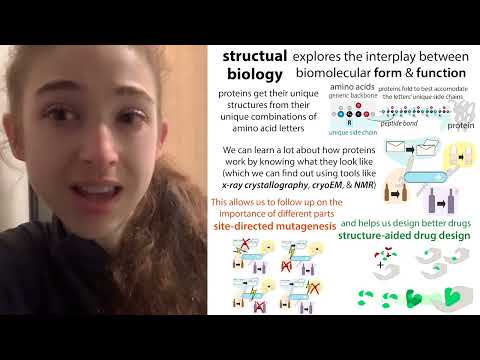 0 Views |
blog form: https://bit.ly/structural_biology_overview
Note: some people in structural biology labs specialize more in specific aspects and so perhaps only deal with the computational or biochemical parts etc., but for many structural biologists, especially during training, they do everything. Or, in some cases, one person will perform the expression and purification and pass it on to another person, potentially in another laboratory, who will take care of the actual structural determination part (collecting and analyzing the data by crystallography, cryo -EM, etc.). Such a collaboration can be a huge win for both! And for the general public because it allows us to capitalize on the diversity of expertise and interests of different laboratories to obtain structures of a wide variety of molecules.
Note: I did my PhD in a structural biology lab (Dr. Leemor Joshua-Tor's lab at Cold Spring Harbor Laboratories) and did some crystallography, but mainly focused on the biochemistry side of things . But I like being structurally adjacent! And I have a deep appreciation for the structural parts of structural biology, although I prefer to leave the determination of structure to others. So I deeply appreciate people who prefer to do that side of things! But back to the story…
So, let's say you solve the structure (determine the position of the atoms making up this protein, etc.). Now what?
You can learn a lot about how a protein works by seeing what it looks like (imagine seeing a photo of an open Swiss Army knife). And you can also discover how a protein might "not work" if you can attach or hide certain parts with another molecule, like a pharmaceutical drug. You can learn a lot from structures and a lot of hypotheses you can generate, but you also need experimental evidence to see if your ideas come to fruition. Thus, a large part of structural biology consists of carrying out biochemistry and biophysics type experiments in the laboratory (as well as sometimes cellular experiments, often in collaboration with other laboratories) to test them.
Using molecular biology, we can use site-directed mutagenesis to introduce specific mutations into genetic recipes for making proteins, thereby introducing changes into the resulting proteins. We can make changes to certain parts of the protein that, depending on the structure, are important for something, and then see if that messes up that "something" (but make sure you don't just mess up protein folding or something else in the process !)
⠀
Going back to our Swiss army knife analogy, it's like if you saw a corkscrew and you wanted to stop people from uncorking the bottles, you could file down the tip of the screw so people couldn't uncork the bottles. insert into the cap.
These structure-directed mutations can therefore help us understand how molecules work. And they can also help us see how we might stop them from working! We might want to do this to attenuate an overactive protein that causes disease or to inactivate a viral protease (protein cutter) to stop it from producing more viruses.
You won't be able to mutate* a protein in a real person, but you can introduce pharmaceutical drugs that bind to the site! And knowing what that site looks like can help.
In the same way that you might design a "cap" that covers the pointed tip of the corkscrew, if you can see what a protein's "active site" looks like, the part where the protein "does things" ( for example the place in a virus) protease where it attaches to proteins and cuts them), you can better design a drug that binds to them and blocks them. Instead of designing from scratch, scientists often start by using crystallography to screen pre-existing drugs (some of which are already approved to treat other diseases) via compound library screening or screening elements (a screening of fragments). Once they get results, they can then look closely at how it interacts with the protein and how they might modify the molecule to bind better. More information here: https://bit.ly/mproinhibitors
Finished in comments
Please take the opportunity to connect and share this video with your friends and family if you find it useful.











