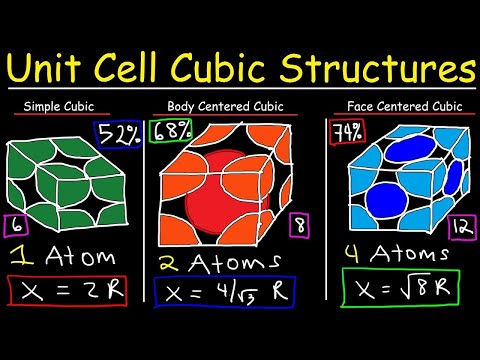
Unit cell chemistry Simple cubic, Body-centered cubic, Face-centered cubic crystal lattice structure
| Channel | Publish Date | Thumbnail & View Count | Download Video |
|---|---|---|---|
| | Publish Date not found |  0 Views |
This chemistry video tutorial provides a basic introduction to unit cell and crystal lattice structures. It highlights the main differences between simple cubic unit cell, body-centered cubic structure and face-centered cubic structure in tabular form. The full version of this video provides the number of atoms per unit cell/coordination number, atomic packing factor/fractional volume efficiency, and formulas for calculating the edge length of each unit cell which can be helpful in calculating the density of the crystal. structure given the atomic radius and vice versa. This video is full of information.
Access the full 45 minute video:
https://www.patreon.com/MathScienceTutor
Direct link to full video:
https://bit.ly/3k0pXlP
Chemistry PDF Worksheets:
https://www.video-tutor.net/chemistry-basic-introduction.html
_________________________________
Full 45 minute video on YouTube:
https://www.youtube.com/watch?v9Ba8MrubcPg
Join the YouTube Membership Program:
https://bit.ly/46xaQTR
Please take the opportunity to connect and share this video with your friends and family if you find it useful.